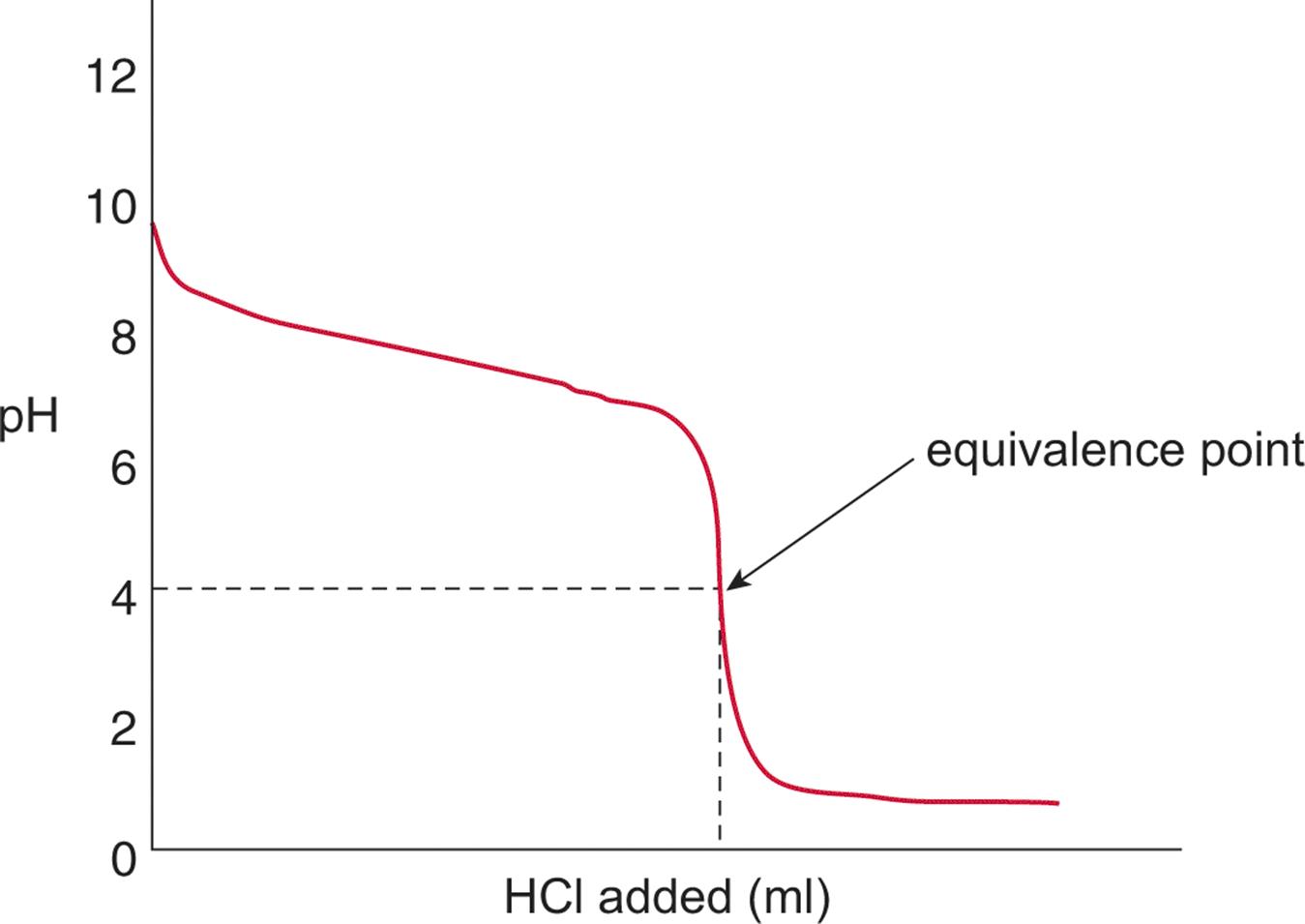
Titration Curve Of Nh3 And Hcl . the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. there are two basic types of acid base titrations, indicator and potentiometric. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. The way you normally carry out a titration involves adding the acid to the alkali. a summary of the important curves. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line. In the strong acid titration,. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions.
from schoolbag.info
In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. there are two basic types of acid base titrations, indicator and potentiometric. a summary of the important curves. In the strong acid titration,. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The way you normally carry out a titration involves adding the acid to the alkali.
Titration and Buffers Acids and Bases Training MCAT General Chemistry Review
Titration Curve Of Nh3 And Hcl a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. there are two basic types of acid base titrations, indicator and potentiometric. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. a summary of the important curves. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the.
From chart-studio.plotly.com
Titration of Ammonia (NH3) with 1.0M HCl line chart made by Ballj4144 plotly Titration Curve Of Nh3 And Hcl there are two basic types of acid base titrations, indicator and potentiometric. In the strong acid titration,. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line. In an indicator based titration you add another chemical that changes color at the ph equal to the. Titration Curve Of Nh3 And Hcl.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Curve Of Nh3 And Hcl In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. a summary of the important curves. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. The. Titration Curve Of Nh3 And Hcl.
From www.chegg.com
Solved Which Of The Following Accurately Describes The Sp... Titration Curve Of Nh3 And Hcl In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. a summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. In the strong acid titration,. the corresponding curve. Titration Curve Of Nh3 And Hcl.
From www.chegg.com
Solved Titration of ammonia with HCl This graph shows the Titration Curve Of Nh3 And Hcl The way you normally carry out a titration involves adding the acid to the alkali. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line. a summary of the important curves. the titration curves shown in figure 14.20 illustrate the choice of a suitable. Titration Curve Of Nh3 And Hcl.
From www.youtube.com
What is the chemical equation for titration of HCl + NH3? YouTube Titration Curve Of Nh3 And Hcl The way you normally carry out a titration involves adding the acid to the alkali. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. In the strong acid. Titration Curve Of Nh3 And Hcl.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration Curve Of Nh3 And Hcl The way you normally carry out a titration involves adding the acid to the alkali. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in. Titration Curve Of Nh3 And Hcl.
From www.numerade.com
SOLVED(a) Draw a pH titration curve that represents the titration of 50.0 mL of 0.10 M NH3 by Titration Curve Of Nh3 And Hcl In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. the corresponding curve for the titration of. Titration Curve Of Nh3 And Hcl.
From www.chegg.com
Solved Given the titration curve for a titration between Titration Curve Of Nh3 And Hcl The way you normally carry out a titration involves adding the acid to the alkali. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. In the strong acid titration,. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific. Titration Curve Of Nh3 And Hcl.
From www.chegg.com
Solved Titration of ammonia with HCl This graph shows the Titration Curve Of Nh3 And Hcl the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence. Titration Curve Of Nh3 And Hcl.
From schoolbag.info
Titration and Buffers Acids and Bases Training MCAT General Chemistry Review Titration Curve Of Nh3 And Hcl Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. The way you normally carry out a titration involves adding the acid to the alkali. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a. Titration Curve Of Nh3 And Hcl.
From www.researchgate.net
Equilibrium fraction of total ammonia, nitric acid, and hydrochloric... Download Scientific Titration Curve Of Nh3 And Hcl In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. The way you normally carry out a titration involves adding the acid to the alkali. In the strong acid titration,. a summary of the important curves. Hydrochloric acid, hcl, a. Titration Curve Of Nh3 And Hcl.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free download ID5976807 Titration Curve Of Nh3 And Hcl a summary of the important curves. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line.. Titration Curve Of Nh3 And Hcl.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free download ID5976807 Titration Curve Of Nh3 And Hcl the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. In the strong acid titration,. The way you normally carry out a titration involves adding the acid to the alkali. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the. Titration Curve Of Nh3 And Hcl.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Of Nh3 And Hcl there are two basic types of acid base titrations, indicator and potentiometric. The way you normally carry out a titration involves adding the acid to the alkali. In the strong acid titration,. a summary of the important curves. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl,. Titration Curve Of Nh3 And Hcl.
From www.chegg.com
Solved QUESTION 43 Which graph best represents the titration Titration Curve Of Nh3 And Hcl the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a dashed line. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. Hydrochloric acid, hcl, a strong acid, will. Titration Curve Of Nh3 And Hcl.
From www.numerade.com
SOLVED Steps plz 2. Given the titration curve for a titration between ammonia, NH3, and Titration Curve Of Nh3 And Hcl a summary of the important curves. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. there are two basic types of acid base titrations, indicator and potentiometric. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with 0.200 m hcl is shown as a. Titration Curve Of Nh3 And Hcl.
From www.researchgate.net
Titration curves of hydrochloricacidbased electrograining solution... Download Scientific Titration Curve Of Nh3 And Hcl a summary of the important curves. Hydrochloric acid, hcl, a strong acid, will react with ammonia, nh3, a weak base, to form aqueous ammonium chloride, nh4cl, according to the. In an indicator based titration you add another chemical that changes color at the ph equal to the equivalence point, when the acid and base are in stoichiometric proportions. In. Titration Curve Of Nh3 And Hcl.
From ceckgokq.blob.core.windows.net
Titration Curve In Biochemistry at William Speece blog Titration Curve Of Nh3 And Hcl The way you normally carry out a titration involves adding the acid to the alkali. a summary of the important curves. In the strong acid titration,. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. the corresponding curve for the titration of 50.0 ml of 0.100 m \(naoh\) with. Titration Curve Of Nh3 And Hcl.